World-class FEM of the spine
With a world-class FEM (Finite Element Model) of the spine and ability to simulate ASTM/ISO testing standards, we are helping small-medium-large spine implant developers accelerate path to market.
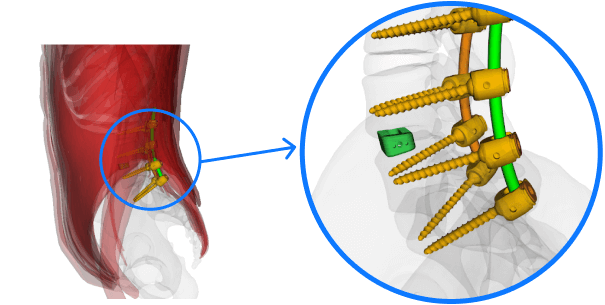
- Test various design concepts in short cycle times
- Perform scientifically backed in silico clinical evaluations to support your claims

Virtual ASTM F543 — Test Methods for Metallic Bone Screws
Validated simulation testing that gives real world results.
Visit ![]() website to purchase screw test simulations
website to purchase screw test simulations
Insertion Torque
Insertion Torque measurement into virtual ![]() block
block
according to ASTM F543-A2
Axial Pullout Strength – FDA Qualified
Pullout force measurement from virtual ![]() block
block
according to ASTM F543-A3
Perform Virtual Mechanical Tests on any Medical Device
Dental implant

ISO 14801
DENTISTRY — IMPLANTS — DYNAMIC LOADING TEST FOR ENDOSSEOUS DENTAL IMPLANTS

ISO 13498
DENTISTRY — TORSION TEST OF IMPLANT BODY/CONNECTING PART JOINTS OF ENDOSSEOUS DENTAL IMPLANT SYSTEMS
Elbow

ASTM F2887
Standard Specification for Total Elbow Prostheses
Exoprosthesis

ISO 10328
Structural testing of lower-limb prostheses – Requirements and test methods
Finger

ASTM F1781
Standard Specification for Elastomeric Flexible Hinge Finger Total Joint Implants.
Hip joint implant

ISO 7206-4, ISO 7206-6, ISO 7206-8, ISO 7206-9, ISO 7206-10,ISO 7206-12, ISO 7206-13
IMPLANTS FOR SURGERY — PARTIAL AND TOTAL HIP-JOINT PROSTHESES

ASTM F2580
Standard Practice for Evaluation of Modular Connection of Proximally Fixed Femoral Hip Prosthesis

ASTM F2345
Standard test methods for determination of static and cyclic fatigue strength of ceramic modular femoral heads

ASTM F2009
Standard Test Method for Determining the Axial Disassembly Force of Taper Connections of Modular Prostheses

ASTM F1820
Standard Test Method for Determining the Forces for Disassembly of Modular Acetabular Devices

ISO 21535
NON-ACTIVE SURGICAL IMPLANTS — JOINT REPLACEMENT IMPLANTS — SPECIFIC REQUIREMENTS FOR HIP-JOINT REPLACEMENT IMPLANTS

ISO 11491
IMPLANTS FOR SURGERY — DETERMINATION OF IMPACT RESISTANCE OF CERAMIC FEMORAL HEADS FOR HIP JOINT PROSTHESES

ASTM F2996
Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems
Knee implant

ASTM F1223
Standard Test Method for Determination of Total Knee Replacement Constraint

ASTM F2724
Standard Test Method for Evaluating Mobile Bearing Knee Dislocation.

ASTM F2083
Standard Specification for Knee Replacement Prosthesis

ISO 14879
IMPLANTS FOR SURGERY — TOTAL KNEE-JOINT PROSTHESES — PART 1: DETERMINATION OF ENDURANCE PROPERTIES OF KNEE TIBIAL TRAYS

ISO 21536
NON-ACTIVE SURGICAL IMPLANTS — JOINT REPLACEMENT IMPLANTS — SPECIFIC REQUIREMENTS FOR KNEE-JOINT REPLACEMENT IMPLANTS

ASTM F1800
Standard Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements

ASTM F3334
Standard Practice for Finite Element Analysis (FEA) of Metallic Orthopaedic Total Knee Tibial Components

ASTM F2777
Standard Test Method for Evaluating Knee Bearing (Tibial Insert) Endurance and Deformation Under High Flexion

ASTM F2723
Standard Test Method for Evaluating Mobile Bearing Knee Tibial Baseplate/Bearing Resistance to Dynamic Disassociation

ASTM F2722
Standard Practice for Evaluating Mobile Bearing Knee Tibial Baseplate Rotational Stops

ASTM F3141
Standard Guide for Total Knee Replacement Loading Profiles

ASTM F3161
Standard Test Method for Finite Element Analysis (FEA) of Metallic Orthopaedic Total Knee Femoral Components under Closing Conditions

ASTM F3140
Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Unicondylar Knee Joint Replacements

ISO 14243-5
IMPLANTS FOR SURGERY — WEAR OF TOTAL KNEE PROSTHESES — PART 5: DURABILITY PERFORMANCE OF THE PATELLOFEMORAL JOINT

ASTM F1814
Standard Guide for Evaluating Modular Hip and Knee Joint Components
Osteosynthesis

ASTM F384
Standard Specifications and Test Methods for Metallic Angled Orthopedic Fracture Fixation Devices.

ASTM F543
Standard Specification and Test Methods for Metallic Medical Bone Screws.

ASTM F1541
Standard Specification and Test Methods for External Skeletal Fixation Devices.

ISO 9585
IMPLANTS FOR SURGERY — DETERMINATION OF BENDING STRENGTH AND STIFFNESS OF BONE PLATES

ASTM F382
Standard Specification and Test Method for Metallic Bone Plates

ASTM F1264
Standard Specification and Test Methods for Intramedullary Fixation Devices.

ASTM F564
Standard Specification and Test Methods for Metallic Bone Staples.

ASTM F2180
Standard Specification for Metallic Implantable Strands and Cables.
Spinal implant

ASTM F2193
Standard Specifications and Test Methods for Components Used in the Surgical Fixation of the Spinal Skeletal System

ASTM F1798
Standard Test Method for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants

ASTM F1717
Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model.

ASTM F2077
Test Methods For Intervertebral Body Fusion Devices

ASTM F2346
Standard Test Methods for Static and Dynamic Characterization of Spinal Artificial Discs

ASTM F2267
Standard Test Method for Measuring Load Induced Subsidence of Intervertebral Body Fusion Device Under Static Axial Compression

ISO 12189
Spine corpectomy model with anterior support fatigue testing of spinal constructs using an anterior supported model. The test method is intended for implants being hinged or too flexible to be loaded according to ASTM F1717″

ASTM F2624
Standard Test Method for Static, Dynamic, and Wear Assessment of Extra-Discal Spinal Motion Preserving Implants.

ASTM F2790
Standard Practice for Static and Dynamic Characterization of Motion Preserving Lumbar Total Facet Prostheses

ASTM F2706
Standard Test Methods for Occipital-Cervical and Occipital-Cervical-Thoracic Spinal Implant Constructs in a Vertebrectomy Model.

ASTM F3295
Standard Guide for Impingement Testing of Total Disc Prostheses
Vascular implant

“ISO 25539-1, ISO 25539-2”
CARDIOVASCULAR IMPLANTS — ENDOVASCULAR DEVICES — PART 2: VASCULAR STENTS

ASTM F2079
Standard Test Method for Measuring Intrinsic Elastic Recoil of Balloon-Expandable Stents

ASTM F3067
Standard Guide for Radial Loading of Balloon-Expandable and Self-Expanding Vascular Stents

ASTM F2394
Standard Guide for Measuring Securement of Balloon Expandable Vascular Stent Mounted on Delivery System

ISO 7198
CARDIOVASCULAR IMPLANTS AND EXTRACORPOREAL SYSTEMS — VASCULAR PROSTHESES — TUBULAR VASCULAR GRAFTS AND VASCULAR PATCHES

ASTM F2477
Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents

ASTM F2606
Standard guide for three point bending of balloon expandable vascular stents and stent systems.

ASTM F2942
Standard Guide for in vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents

ASTM F2514
Standard Guide for Finite Element Analysis (FEA) of Metallic Vascular Stents Subjected to Uniform Radial Loading
Wrist implant

ASTM F1357
Standard Specification for Articulating Total Wrist Implants
